Author: Almudena González Domínguez
The PICO approach (Population, Intervention, Comparator, Outcomes) has become the cornerstone of the clinical assessment process within the new European framework for the Joint Clinical Assessment (JCA) of health technologies.
Why is it so relevant?
PICO is not just a traditional methodological tool for clinical research. Within the JCA context, it acts as a bridge between the health policy questions of EU Member States and the evidence requirements for health technology developers — in other words, it serves as a common European methodological framework. This approach allows countries with diverse healthcare systems to address the same research questions, thereby enhancing the robustness of joint reports and the credibility of decision-making. Its true value lies in fostering a shared evaluation culture across Europe, where every decision is supported by comparable and relevant evidence.
Key steps in defining PICO within the JCA process
1. Start from health policy questions: PICO translates national needs into structured and comparable research questions.
2. Engage all Member States: Through PICO surveys, input is collected on population, intervention, comparators, and outcomes.
3. Integrate diverse perspectives: Integrate diverse perspectives: not only authorities, but also patients, clinicians, and experts provide key information to refine definitions.
4. Consolidate without losing relevance: National proposals are harmonized into the smallest possible number of PICOs, ensuring that no need is left out.
5. Establish the assessment framework: Each resulting PICO defines which data must be requested from the developer and how the joint report will be structured.
From theory to practice
In spring 2024, the JCA Subgroup conducted six PICO pilot exercises: three for medicinal products and three for medical devices. These case studies (publicly available on the European Commission website) illustrate how the methodology translates into concrete scenarios, serving as a reference for future processes.
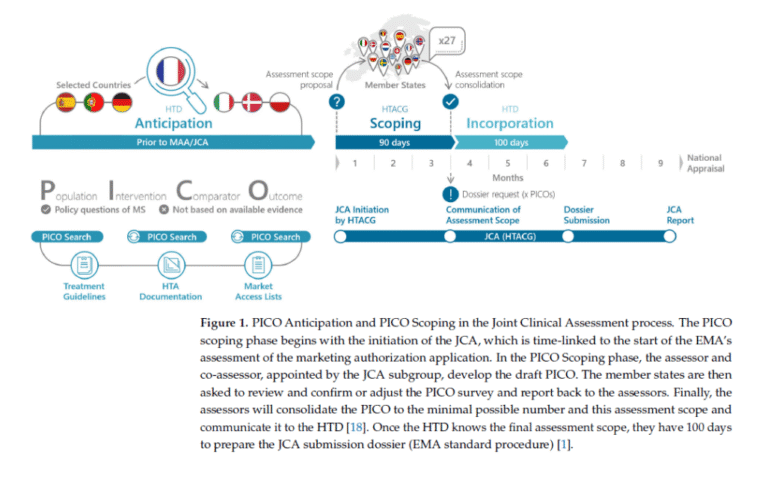
Furthermore, developers will need to anticipate the PICO based on available information. This information generally originates from Health Technology Assessment Agencies, following the general implementation scheme shown in the figure below.
Source: Eberle K. et al., 2025
Additional Information of Interest: PICO vs. PECO — Choosing the Right Approach According to the Type of Evidence
Although PICO (Population, Intervention, Comparator, Outcomes) is the most widely used format in clinical assessments—particularly within the European Joint Clinical Assessment (JCA) framework—the PECO approach (Population, Exposure, Comparator, Outcomes) is more suitable for observational studies or in contexts where there is no active intervention but rather a natural or environmental exposure. The key difference lies in the type of evidence being sought: while PICO focuses on controlled clinical trials, PECO allows the exploration of associations in cohort or case-control studies. This distinction is relevant because, in the evaluation of health technologies—especially medical devices or non-pharmacological interventions—it may be necessary to rely on observational evidence. Recognizing when to apply PICO or PECO ensures that assessment questions are aligned with the type of evidence available, thereby improving the quality and applicability of joint reports and facilitating subsequent processes of health technology assessment and funding.
References
Cumpston M, Flemyng E. Chapter II: Planning a Cochrane Review [last updated August 2023]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane; 2024. Available from: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-ii
Eberle, K.; Hagemann, L.M.; Schweitzer, M.K.; Justl, M.; Maurer, J.; Carls, A.; Reuter, E.-M. The PICO Puzzle: Can Public Data Predict EU HTA Expectations for All EU Countries? J. Mark. Access Health Policy 2025, 13, 32. https://doi.org/10.3390/jmahp13030032
European Commission. HTA JCA Scoping Process. Brussels: Directorate-General for Health and Food Safety; 2023 [cited 2025 Oct 7]. Available from: https://health.ec.europa.eu/document/download/7be11d76-9a78-426c-8e32-79d30a115a64_en?filename=hta_jca_scoping-process_en.pdf