Author: María Merino
A systematic review is a study that—using an explicit, reproducible protocol—identifies, selects, critically appraises, and rigorously synthesizes all the available evidence on a specific research question (with meta-analysis when appropriate). It is considered the highest level of evidence (Level I) because it integrates results from multiple studies.
What does a systematic review contribute? (1,2)
Systematic reviews emerged from the need to ensure that decisions affecting people’s lives are based on a complete and up-to-date understanding of the relevant evidence. With scientific literature growing continuously, it is impossible for health decision-makers to appraise the vast number of primary studies to make the most appropriate choices.
A systematic review seeks to gather all empirical evidence that meets pre-specified eligibility criteria to answer a specific research question. It uses explicit and systematic methods designed to minimize bias in the identification, selection, and interpretation of evidence, thereby delivering more reliable findings on which conclusions and decisions can be based. Systematic reviews can provide an up-to-date summary of the state of scientific knowledge on an intervention, a diagnostic test, or another health-care topic.
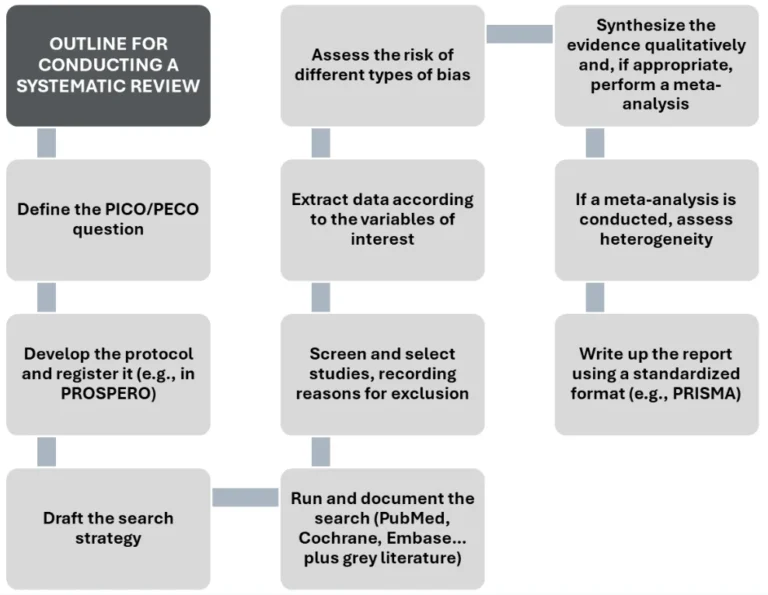
However, conducting a systematic review is not free of challenges, and achieving high quality is often time- and labour-intensive. In fact, the usefulness of any systematic review lies in its quality; therefore, one must understand in detail all the required steps and the key considerations at each stage (Figure 1).
Figure 1. Outline for conducting a systematic review.
Source: authors’ elaboration based on IOM (Institute of Medicine) (2011) and Higgins et al. (2024) (1,2)
Key tools to ensure quality: PROSPERO and PRISMA
In this context, two tools are essential to safeguard transparency, quality, and reproducibility in systematic reviews: the PROSPERO registry (International Prospective Register of Systematic Reviews) (3) and the PRISMA guide (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (4).
On the one hand, PROSPERO is one of the main international databases where authors publicly register their review protocol before starting. This step—now increasingly required by journals—helps avoid duplication of effort, increases process transparency, and reduces the risk of reporting bias, since methods and objectives are specified in advance. The registry also enables other researchers to check ongoing reviews and assess consistency between the registered protocol and the published results.
On the other hand, the PRISMA guideline sets out criteria to improve transparency, completeness, and quality in reporting systematic reviews and meta-analyses. Its core element is the PRISMA checklist, a 27-item list indicating what information should be included in each section of the report—from the title and objectives to methods, results, discussion, and conclusions.
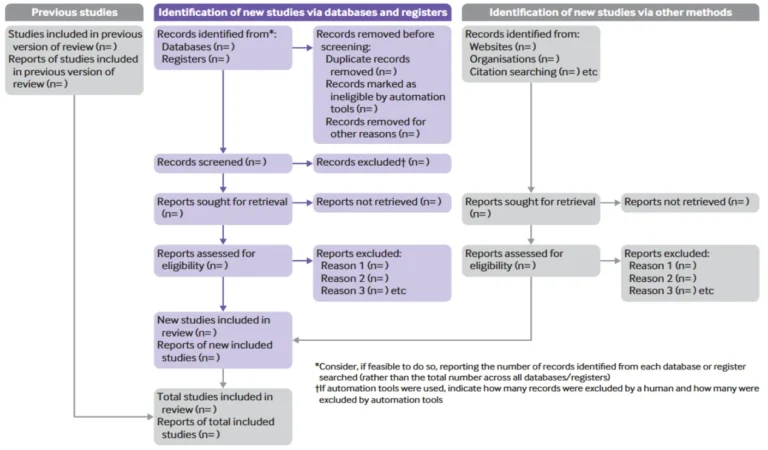
PRISMA also includes the well-known flow diagram (Figure 2), which visually represents the process of identifying, selecting, excluding, and including studies. This diagram has become a standard in the scientific literature because it allows readers to grasp briefly the thoroughness of the literature search. Following PRISMA—particularly the checklist—facilitates critical appraisal by reviewers and readers, increases credibility and comparability of results, and promotes more open and reproducible science.
Figure 2. PRISMA 2020 flow diagram template for systematic reviews.
Source: Page et al., 2021 (4).
PROSPERO and PRISMA are essential methodological pillars to ensure that systematic reviews are rigorous, transparent, and useful for evidence-based decision-making.
Illustrative example
Systematic reviews, together with meta-analyses, can mark turning points in health-care practice. An illustrative example is a systematic review with meta-analyses conducted in the first months after the declaration of the COVID-19 pandemic.
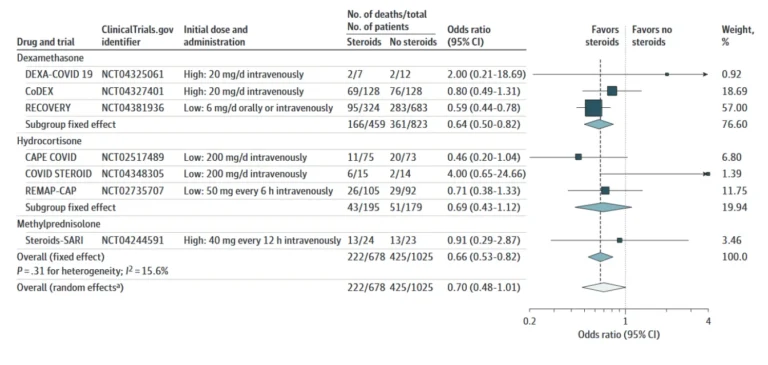
Led by The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, this study analysed 7 randomized controlled trials (total 1,703 patients) to assess whether corticosteroids (e.g., dexamethasone) could reduce mortality in critically ill COVID-19 patients. The results suggested that systemic corticosteroids, compared with usual care or placebo, were associated with lower 28-day all-cause mortality, with a summary OR of 0.66 (95% CI: 0.53–0.82; p < .001) under a fixed-effect meta-analysis, and a summary OR of 0.70 (95% CI: 0.48–1.01; p = .053) under a random-effects meta-analysis (Figure 3) (5). This evidence led to global recommendations in favour of corticosteroids for severe cases and against their use in non-severe patients (6).
Figure 3. Association between corticosteroids and 28-day all-cause mortality in each trial, overall, and according to corticosteroid drug.
Source: The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, 2020 (5).
References
1. IOM (Institute of Medicine). Finding What Works in Health Care: Standards for Systematic Reviews [Internet]. Washington, D.C.: National Academies Press; 2011. Disponible en: https://www.nap.edu/catalog/13059
2. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editores. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024) [Internet]. Cochrane; 2024. Disponible en: www.cochrane.org/handbook
3. Centre for Reviews and Dissemination. Centre for Reviews and Dissemination, University of York. [citado 21 de octubre de 2025]. PROSPERO: International prospective register of systematic reviews. Disponible en: https://www.crd.york.ac.uk/prospero/
4. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 2021;372:n71.
5. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330-41.
6. World Health Organization. Corticosteroids for COVID-19 [Internet]. Geneva: World Health Organization; 2020 [citado 21 de octubre de 2025] p. 32. (Clinical management of COVID-19 – Module 3). Disponible en: https://www.who.int/docs/default-source/coronaviruse/module-3-corticosteroid-therapy-and-covid19.pdf